In the modern world, we are surrounded by a myriad of personal care products promising to make us look and feel our best. From shampoos and soaps to toothpaste and body washes, these products often contain a multitude of ingredients, many of which we are not familiar with. A class of ingredients that we do need to be aware of, though, are Sodium Lauryl Sulfate (SLS) and Sodium Laureth Sulfate (SLES). As incredibly common components of many personal care items, understanding what SLS and SLES are, and how they affect our health and the environment, is crucial.
Sodium Lauryl Sulfate is widely used for its powerful cleansing abilities, while Sodium Laureth Sulfate, an ethoxylated derivative of SLS, is prized for its ability to create a rich lather and cleanse the skin and hair. You would think that these ingredients, used in so many products, would be completely harmless, right? Well… not quite – these ingredients bring up some health concerns and environmental impacts that many consumers are unaware of. In this blog, we will unpack the world of Sodium Lauryl Sulfate and Sodium Laureth Sulfate, exploring their chemical properties, uses, health implications, and the effects they have on our environment.
By shedding light on these aspects, we aim to empower you with the knowledge needed to make more informed decisions about the products you use daily. Whether you’re a conscientious consumer seeking safer alternatives or simply curious about what’s in your shampoo, this blog will provide valuable insights into why you should care about Sodium Lauryl Sulfate and Sodium Laureth Sulfate and what steps you can take to minimize their impact on your health and the planet.
So What Are They?
Sodium Lauryl Sulfate (SLS)
Sodium Lauryl Sulfate (SLS) is a synthetic detergent and surfactant commonly used in a variety of personal care and cleaning products. It is known for its potent ability to remove oil and dirt, which makes it an effective cleaning agent. However, its strong cleansing properties also make it more likely to cause skin irritation.

Sodium Laureth Sulfate (SLES)
Sodium Laureth Sulfate (SLES) is an ethoxylated derivative of SLS, formed by reacting SLS with ethylene oxide. This process creates a compound that is less irritating to the skin compared to its precursor, making it a popular choice in formulations that require mildness along with effective cleansing properties.
Chemical Composition and Origin
- Sodium Lauryl Sulfate: Composed of a hydrophobic tail derived from lauryl alcohol and a hydrophilic sulfate group, SLS effectively reduces the surface tension of water, enabling it to trap oil and dirt, which can then be rinsed away.
- Sodium Laureth Sulfate: Similarly structured, SLES includes an ethoxy group in its hydrophilic head, making it less harsh. However, the ethoxylation process introduces concerns about potential contaminants such as 1,4-dioxane, a byproduct classified as a probable human carcinogen.
Common Uses in Personal Care Products
SLS and SLES are valued in the personal care industry for their ability to produce a foamy lather that enhances the user experience. Their primary role as surfactants makes them effective cleaning agents, commonly found in:
- Shampoos: Providing the foamy texture that helps distribute the product through hair, ensuring thorough cleaning.

- Body Washes and Soaps: Creating a lather that makes skin feel clean.
- Toothpaste: Assisting in the foaming action that helps remove food particles and plaque from teeth.
- Facial Cleansers: Helping to remove makeup, oil, and impurities from the skin.
Beyond personal care, SLS and SLES are also used in household cleaning products, where their surfactant properties help lift dirt and grease from surfaces.
How Do They Work?
SLS and SLES are highly valued in the personal care industry due to their roles as surfactants. Surfactants are compounds that lower the surface tension between two substances, such as oil and water, making it easier to remove dirt and oil from surfaces. Understanding how SLS and SLES function can provide insight into why they are so widely used and why their presence in products warrants consideration.
Mechanism of Action as Surfactants
- SLS: SLS molecules have a hydrophobic tail and a hydrophilic head. When SLS is added to a product, its molecules arrange themselves at the interface between water and oil, with their hydrophobic tails embedded in the oil and their hydrophilic heads in the water. This arrangement reduces the surface tension of the water, allowing it to mix with oils and dirt more effectively.
- SLES: SLES functions similarly, but its ethoxylated structure makes it less likely to cause irritation while still being effective at breaking down oils and dirt.
When you apply a product containing SLS or SLES, this surfactant action helps to emulsify and lift away oils, dirt, and impurities from your skin or hair, which can then be rinsed away with water.
Why They are Used in Products
The surfactant properties of SLS and SLES make them popular ingredients in many formulations due to several benefits:
- Foaming Ability: Both SLS and SLES create a rich, foamy lather, which enhances the sensory experience of using a product. Consumers often associate foam with cleanliness and efficacy.

- Effective Cleansing: Their ability to break down oils and dirt ensures that products containing SLS and SLES are effective at cleaning skin and hair.
- Stability and Versatility: Both compounds are stable across a wide range of pH levels and temperatures, meaning they can be used in various formulations without compromising their effectiveness. They also work well in combination with other ingredients, making them versatile components in complex product formulations.
While these benefits explain why SLS and SLES are so widely used, it is important to consider their potential drawbacks. Despite their effectiveness and popularity, both can pose health risks, particularly for individuals with sensitive skin or pre-existing skin conditions. Additionally, the production and use of SLS and SLES have environmental implications that are important to understand. These considerations will be explored further in the following sections of this blog.
Potential Health Concerns
While Sodium Lauryl Sulfate and Sodium Laureth Sulfate are widely used for their cleansing and foaming properties, they are not without potential health risks.
Skin Irritation and Sensitivity
One of the most common health concerns associated with SLS and SLES is their potential to cause skin irritation and sensitivity. SLS is known for being a stronger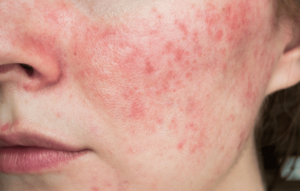 irritant, while SLES, though milder due to its ethoxylation process, can still strip away natural oils from the skin, leading to dryness and irritation. This is particularly problematic for individuals with sensitive skin or conditions such as eczema and dermatitis.
irritant, while SLES, though milder due to its ethoxylation process, can still strip away natural oils from the skin, leading to dryness and irritation. This is particularly problematic for individuals with sensitive skin or conditions such as eczema and dermatitis.
- Dermatological Reports: Numerous studies have highlighted cases where prolonged use of products containing SLS or SLES has led to skin irritation. Dermatologists often advise individuals with sensitive skin to avoid products with these ingredients to minimize the risk of adverse reactions.
- Populations Most at Risk: People with pre-existing skin conditions, children, and individuals with a history of allergies may be more susceptible to the irritant effects of SLS and SLES. For these populations, even low concentrations can cause significant discomfort.
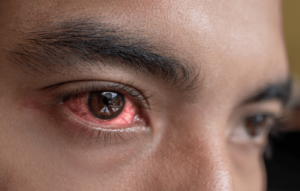
Eye Irritation and Damage
Both SLS and SLES can pose risks to eye health. When they come into contact with the eyes, they can cause irritation and redness. This is a concern for products like shampoos and facial cleansers that are used near the eye area.
Research has shown that SLS can cause moderate to severe eye irritation, while SLES, though milder, can still cause mild to moderate irritation. Prolonged exposure or accidental splashes in the eyes can lead to more severe irritation and, in rare cases, damage to the eye’s surface.
Respiratory Issues
Inhalation of products containing SLS or SLES can lead to respiratory issues. This is especially concerning for individuals with asthma or other respiratory conditions.
When SLS or SLES is used in aerosolized products, fine particles can be inhaled, potentially causing respiratory irritation. This is a significant risk for people using such products in poorly ventilated areas or in close proximity to the face.
While one-time exposure may only be a minor issue, if at all, for most, it’s the idea of near-constant exposure to these chemicals in a world that is already putting a lot of strain on our bodies that is the greater concern.
Understanding these potential health concerns highlights the importance of being aware of the ingredients in the products you use. While SLS and SLES are effective and widely used, we have to also consider these risks and weigh them against the benefits, particularly when there are other less-problematic options.
Environmental Impact
In addition to potential health concerns, Sodium Lauryl Sulfate (SLS) and Sodium Laureth Sulfate (SLES) also pose significant environmental risks. Understanding these impacts is essential for making mindful choices about the products we use and their broader effects on the planet.
Biodegradability of SLS and SLES
Both SLS and SLES are partially biodegradable, meaning they can break down over time in the environment. However, the rate and completeness of their biodegradation can vary, and in some cases, they may persist in the environment longer than desired.
- SLS: Generally considered to be more readily biodegradable than SLES, SLS can still pose risks if it accumulates in large quantities.
- SLES: The ethoxylation process that makes SLES milder also makes it less readily biodegradable. The presence of contaminants like 1,4-dioxane can further complicate its environmental footprint.
Effects on Aquatic Life
The presence of SLS and SLES in water bodies can be harmful to aquatic organisms.  These chemicals can reduce the surface tension of water, disrupting the natural behavior of aquatic life and potentially leading to toxic effects.
These chemicals can reduce the surface tension of water, disrupting the natural behavior of aquatic life and potentially leading to toxic effects.
- Toxicity Levels: Studies have shown that both SLS and SLES can be toxic to fish and other marine life at certain concentrations. They can cause damage to gills and kidneys, disrupt normal biological functions, and even lead to mortality in severe cases.
- Accumulation and Long-Term Impact: Continuous discharge of these surfactants into water bodies, especially in areas near manufacturing plants or heavily populated regions, can lead to accumulation and long-term environmental damage.
Pollution from Manufacturing and Disposal
The production and disposal of SLS and SLES contribute to environmental pollution. The manufacturing process, particularly the ethoxylation of SLES, can release harmful byproducts into the environment. 
- Manufacturing Footprint: The production of SLS and SLES involves significant energy consumption and the use of hazardous chemicals. Waste products and emissions from these processes can contribute to air and water pollution.
- Disposal Concerns: When products containing SLS and SLES are washed down the drain, they enter wastewater systems. While wastewater treatment plants can remove some of these chemicals, not all facilities are equipped to handle them effectively, leading to their release into natural water bodies.
Alternatives to Sodium Lauryl Sulfate and Sodium Laureth Sulfate
For those looking to avoid SLS and SLES, there are several alternatives available. These alternatives can offer similar benefits without the associated health and environmental risks.
Natural and Less Harmful Surfactants
- Coconut Oil-Derived Surfactants: Ingredients like cocamidopropyl betaine, derived from coconut oil, provide gentle cleansing and foaming
 without the harshness of SLS or SLES.
without the harshness of SLS or SLES. - Decyl Glucoside, Coco Glucoside: These mild surfactants are derived from plant-based sources like corn glucose and coconut oil, offering effective cleansing with a lower risk of irritation and environmental impact.
Comparing Effectiveness and Safety of Alternatives
While alternatives to SLS and SLES may not produce as much lather, they can still provide effective cleansing without the associated risks.
- Performance in Cleansing: Alternatives like decyl glucoside and coco glucoside can effectively remove dirt and oils while being gentler on the skin.
- Environmental Benefits: These alternatives are typically more biodegradable and less toxic to aquatic life, making them a better choice for environmentally conscious consumers.
Consumer Tips
To make safer choices for both health and the environment, consider the following tips:
- How to Identify SLS and SLES on Product Labels: you can easily find “sodium lauryl sulfate,” “sodium laureth sulfate,” and their synonyms on ingredient lists of products.
- Choosing SLS- and SLES-Free Products: Many brands offer products free from these surfactants. Look for labels indicating “SLS-free” or “SLES-free.”
- DIY Personal Care Recipes: For those who prefer homemade solutions, there are many simple recipes available for making personal care products without SLS or SLES.
Final Thoughts
Understanding the potential health and environmental impacts of Sodium Lauryl Sulfate (SLS) and Sodium Laureth Sulfate (SLES) is crucial in today’s world where we have such a wide array of personal care products. These surfactants, while highly effective in providing the cleansing and foaming properties that consumers enjoy, come with some drawbacks that should not be overlooked when so much of the world is suffering from a lack of health.
SLS, known for its potent cleaning power, and SLES, favored for its milder nature and rich lather, both pose risks of skin irritation, eye damage, and respiratory issues, especially for those with sensitive skin or pre-existing conditions. Additionally, their environmental footprint is considerable, with concerns about biodegradability, aquatic toxicity, and pollution from manufacturing processes.
Awareness and education about these ingredients can empower consumers to make better choices. By understanding the differences between SLS and SLES, recognizing their presence in products, and exploring safer alternatives, we can mitigate their impact on our health and the environment. Natural and less harmful surfactants, like those derived from coconut oil or plant-based sources, offer promising substitutes that provide effective cleansing without the associated risks.

As consumers, our choices matter. Opting for products that are free from SLS and SLES, reading ingredient labels carefully, and considering DIY options can contribute to a healthier lifestyle and a more sustainable planet. By making informed decisions, we can enjoy the benefits of personal care products without compromising our well-being or the environment.
All in all, while SLS and SLES are prevalent in many personal care products, their potential health and environmental impacts necessitate careful consideration. By staying informed and choosing alternatives, we can take meaningful steps toward a safer and more environmentally friendly future.



 without the harshness of SLS or SLES.
without the harshness of SLS or SLES.